Author Guidelines
Instructions for Authors
As an international online journal, European Rehabilitation Journal (Eur Rehab J) publishes articles whose interventions cover adult or pediatric rehabilitation activities.
The aim of the journal is to provide new scientific information of high quality and promote the education of clinicians whose area of expertise is rehabilitation.
The Corresponding Author should complete the online Manuscript Submission Form.
By submitting a manuscript in the European Rehabilitation Journal, authors should certify that
-the manuscript is original and not be published elsewhere, not being considered for publication elsewhere, including publicly accessible websites or e-print servers.
-all authors have read and validated the submission.
-clinical trials have been registered in a public trial registry. Please view the ICMJE's page on clinical trial registration for further detail.
-If part of the method or results has already been submitted or submitted for communication at a scientific conference, the authors must specify this in the cover letter and title page by specifying the name of the conference and the date of presentation.
Any change in authorship following the original submission must be justified and agreed to in writing by the affected author(s).
Authors have the option to suggest reviewers. Suggested reviewers should not have common publications with authors or collaborations for the past 3 years. Authors also have the option of disqualifying 2 reviewers for potential conflicts of interest.
Criteria for authorship
In accordance with the ICMJE recommendations defining the role of authors, authorship should be based on all four of the following criteria:
- Substantial contributions to the conception or design of the work; or the acquisition, analysis, or interpretation of data for the work; and
- Drafting the work or revising it critically for important intellectual content; and
- Final approval of the version submitted for publication; and
- Agreement to be accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved.
Fees
The European Rehabilitation Journal (ERJ) operates as a Diamond Open Access journal, meaning that authors do not pay any fees to publish their articles.
Ethics and Transparency
Editors of European Rehabilitation Journal encourage authors to refer to international statements and guidelines designed to improve the accuracy and transparency of clinical research ( EQUATOR Network Resource Center).
All research should follow the recommendations concerning human research that are contained in the Declaration of Helsinki. The Editors reserve the right to reject any manuscript containing studies that do not conform to these recommendations. All manuscripts reporting human research must contain a statement in the text that the institutional review board for human studies approved the protocols and written consent was obtained from the subjects or their surrogates if required by the institutional review board.
European Rehabilitation Journal strongly encourages that all datasets on which the conclusions of the paper rely should be available to readers. A data-sharing statement could be included in the manuscript.
Plagiarism
The European Rehabilitation Journal (ERJ) employs the Similarity Check system powered by CrossRef to ensure the originality and integrity of submitted manuscripts. This advanced tool allows the journal to detect potential instances of plagiarism by comparing the content of manuscripts against a comprehensive database of published works. By utilising this system, the ERJ upholds the highest standards of academic honesty and publication ethics.
Peer-review process
The European Rehabilitation Journal (ERJ) ensures a rigorous evaluation process for all submitted manuscripts. Initially, each manuscript is reviewed by an editor to assess its relevance, originality, and adherence to the journal's scope and guidelines. Manuscripts that meet these criteria are then subjected to a double-blind peer-review process, where both reviewers and authors remain anonymous to each other. This approach guarantees impartiality and fairness while maintaining the highest standards of scientific integrity and quality.
Types of manuscripts
The European Rehabilitation Journal consider for publications following articles format:
- Brief reports are considered for publication. Briefs reports report results concisely when the research question does not require the writing of a full paper. Brief reports should not report preliminary results of a larger study. Brief reports must be limited to 1500 words, up to 3 tables and/or figures and 20 references.
- Case reports are considered for publication. Clinical cases should describe rare situations or new ideas that may have facilitated or greatly improved organizations or patient care.
- Editorial is limited to 1500 words and 10 references. Editorial should highlight key points in correspondence with the editorial policy of the journal.
- Letters to the editor should comment on an article that was published in ERJ with clear argumentation and references. They are limited to 1500 words and 10 references.
- Narrative review are not considered for publication unless it has been invited by the editors. If an author wishes to submit a narrative review, the author should contact the Editorial Board editor@sfphysio.com.
- Original Research (Clinical trials, observational studies, retrospectives studies) including systematic review and Meta-analysis were particularly welcomed. Manuscripts that do not adhere to rigorous methodology will not be considered for publication. Authors should follow the different guidelines according to their study design (CONSORT, PRISMA, STROBE etc).
- Surveys of practices are considered but had low priority for publication.
- Viewpoints can be submitted to ERJ about a particular topic length are not strictly limited but the author should be as concise as possible
- Study Protocol describes plans for conducting research projects and consist of a single article on ERJ. The protocol must:
- Relate to a research study that has not yet generated results.
- Be submitted before or at the very beginning of participants' recruitment or collection of data.
- Meet the same standards for ethics of experimentation and research integrity as the research study. Approval from the relevant ethics body must be obtained prior to submission.
Here is some additional prerequisites depending on study types:
- Protocol of Clinical trials:
- We encourage to register the trial prior to submit your protocol in one of the publicly accessible registries approved by the WHO or ICMJE (International Committee of Medical Journal Editors).
- The name of the registry and the trial or study registration number must be included in the Abstract.
- the SPIRIT schedule of enrollment, interventions, and assessments guideline must be included as a manuscript’s Figure, and a completed SPIRIT checklist must be uploaded as supplementary data.
-
- Protocols of Systematic reviews and meta-analyses:
A completed PRISMA-P checklist must be provided as supplementary data. See PRISMA-P Explanation and Elaboration for more information on completing your checklist.
- Protocols of Systematic reviews and meta-analyses:
- To avoid any bias prior to publication we tend to publish only level 6 protocols.
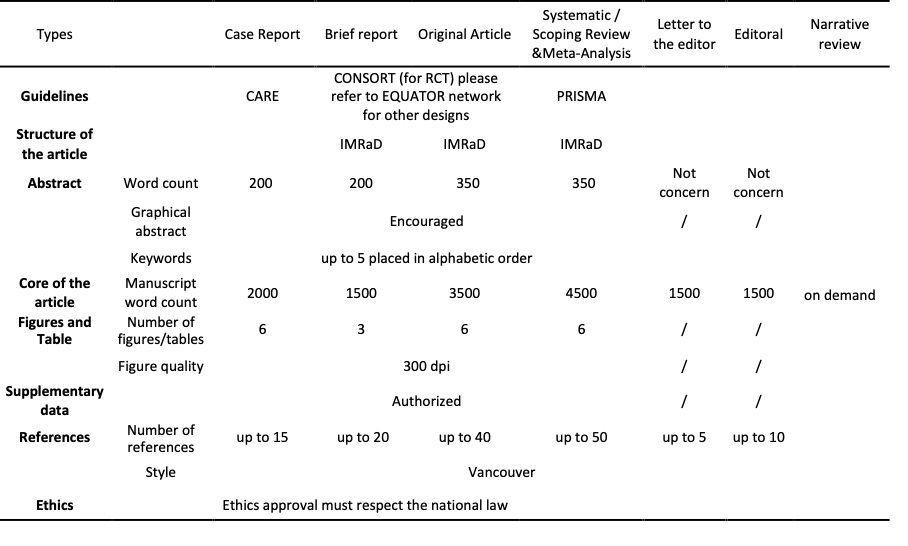
Preparing your manuscript
General information for preparation
- Font large enough for easy reading (e.g., 11 or 12 point). Common fonts should be used (e.g., Arial, Calibri, Helvetica).
- Margins should be no larger than 2.5 centimeters (1 inch)
- Double-spaced text for the main text and references
- Line numbers should be inserted continuously throughout the manuscript.
- Manuscript pages should be numbered consecutively beginning with the title page.
- Never use abbreviations or acronyms in the abstract
The manuscript should be organized into sections following a general order with separated documents to ensure the double-blind peer-review process:
- In a first document (the Title page):
- Title
- Authors and affiliations
- Acknowledgment
- Funding
- A second document should include:
- Abstract + Keywords
- Main Text (figure and table captions could be included in the text)
- References (see below for more information)
- Tables and Figures should be included in separate files
Title page
The title page should include the following items
- Title including if applicable the study design
- A list with the full names, institutional addresses and email for all authors (ORCiD number can be added)
- The name and address of the corresponding author and his email.
- All source(s) of support in the form of grants, gifts, equipment, and/or drugs
- Competing interests
- Authors' contributions
- State the numbers of words counts in the text excluding abstract and references, acknowledgment, figures, etc)
- If there is supplemental material published online, please indicate a sentence (Supplemental material are available on rehab-journal.fr)
Abstract
European Rehabilitation Journal requires a structured abstract for original research.
The abstract should not exceed 250 words and include the following separate section :
- Background
- Objective
- Method
- Results
- Conclusions
- Trials registration
No abstract is needed for Brief reports and clinical cases. Narrative reviews require an unstructured abstract
Keywords
Using MeSH (Medical Subject Headings), list 3 to 5 keywords describing the subject of the manuscript
Main Text
Main text should respect the number of words according to the design (Table).
The manuscripts must be clear, easy to read, and well written.
The introduction should recall the clinical and scientific context, formulate the hypothesis and announce the objective of the study.
In the Methods, authors must announce the design and guidelines followed.
Authors must confirm IRB review and approval or affirm that the protocol is otherwise consistent with the principles of the Declaration of Helsinki.
Authors should also affirm in the Methods that study participants gave informed consent for participation in the study or state that an institutional review-board approved the conduct of the research without explicit consent from the participants.
A statistical paragraph should be present if applicable. Statistical methods must be described and the program used for data analysis, and its source, should be stated. Results must be expressed with their uncertainty about value (Standard deviation, Interquartile Range, or 95% Confidence interval). P-value should be reported for each test, including when there is no significant difference. P-value must be written at a minimum until the second decimal place (eg p=0.057).
All results must be listed without discussion or interpretation. The results can be transcribed in text, Table, or figure. It is important to avoid redundancies.
The discussion should be based on the results based on the most recent data. Authors should include a paragraph "Limitations" in their discussion.
The conclusion must be consistent with the results of the announced hypothesis.
Acknowledgment
This usually follows the Discussion and Conclusions sections. Its purpose is to thank all of the people who helped with the research but did not qualify for authorship. Acknowledge anyone who provided intellectual assistance, technical help (including with writing and editing), or special equipment or materials.
TIP: The International Committee of Medical Journal Editors has detailed guidelines on who to list as an author and who to include in the Acknowledgments that are useful for scientists in all fields.
Funding
You are requested to identify who provided financial support for the conduct of the research and/or preparation of the article and to briefly describe the role of the sponsor(s), if any, in study design; in the collection, analysis, and interpretation of data; in the writing of the report; and in the decision to submit the article for publication. If the funding source(s) had no such involvement, then this should be stated.
References
All references should be edit with the Vancouver style which consists of:
- Citations to someone else's work in the text, indicated by the use of a number.
- A sequentially numbered reference list at the end of the document providing full details of the corresponding in-text reference.
- Authors should plan to create a BibTeX file for the production once the manuscript will be accepted. The BibTeX file can be export directly from bibliographic managers such as Zotero, EndNote, Mendeley or the BibTeX format for each reference can be copy-paste from google scholar.
Additional Guidelines for Review
Review articles can be of two types: systematic review (with or without metanalysis) or scoping review. The systematic and scoping reviews must be a synthesis of evidence, based on a well-defined review question, that is relevant and applicable to rehabilitation clinicians.
The literature search must have been done less than 12 months before the manuscript submission.
Text should include 6 sections: Introduction, Methods, Results, Discussion, Conclusion, and Highlights.
A structured Abstract should be added to the Main Document. Including headings Objective, Design, Data sources, Eligibility criteria for selecting studies, Results, and Summary/Conclusion.
- Word count: up to 4500 words
- Abstract: up to 350 words
- Tables/illustrations: Maximum 6 tables and/or figures
- References: up to 100
- Checklist: Prisma checklist/statement and flowchart (PRIMA or PRISMA-ScR)
- Highlights: 3 to 5 sentences are required
If authors feel their review warrants additional length, tables/figures and/or references, they could consult the editorial office and/or mention the reason in their Cover letter.
Systematic Review and Meta-analysis
The systematic review must focus on a specific clinical question. The title should include ‘a Systematic Review’. Review with registration is more likely to be accepted. Registry and number must be indicated in the manuscript. Authors must complete the PRISMA checklist and the manuscript must include the PRISMA Flow using the guideline for a systematic review (http://prisma-statement.org/PRISMAStatement/).
A meta-analysis may be conducted when possible. A rigorous method will be required in the analysis of the biases of each included study in the meta-analysis, and their heterogeneity. We encourage authors to be assisted by an expert in the field.
Scoping review
For a reminder, a scoping review is useful for examining emerging evidence when it is still unclear and a systematic review cannot be performed. The title should include ‘a Scoping Review’. Authors must complete the PRISMA checklist and the manuscript must include the PRISMA Flow using the guideline for scoping reviews is the PRISMA Scoping Review Extension (https://www.prisma-statement.org/Extensions/ScopingReviews).
If you don't know which choice to make between a systematic review and a scoping review, you can read Munn et al. (https://bmcmedresmethodol.biomedcentral.com/articles/10.1186/s12874-018-0611-x).
To know the methodology of the scoping review, you can read Peters et al.(https://doi.org/10.1097/XEB.0000000000000050.)





